The CLP Regulation (Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging of substances and mixtures) is an EU law that aligns the European system with the Globally Harmonised System (GHS) developed by the United Nations. Its purpose is to ensure that hazards associated with chemicals are clearly communicated through standardized labelling and classification practices across all EU Member States.
CLP replaces the older Dangerous Substances and Dangerous Preparations Directives and works alongside the REACH Regulation (EC No 1907/2006) to ensure the safe use of chemicals, protect human health and the environment, and support free trade through consistent hazard communication.
CLP applies to all chemical substances and mixtures placed on the EU market. This includes candles, diffusers, personal care products and perfumes. It requires manufacturers and importers to:
- Classify substances and mixtures according to their hazards.
- Label them clearly and consistently using standardized elements.
- Package them safely.
What needs to be written on my CLP label?
The required CLP label elements can be found in Section 2.2 of the Safety Data Sheet (SDS). Your label must include, where applicable:
- Hazard pictograms
- Signal word (“Warning” or “Danger”)
- Hazard statement(s) (e.g., H317, H411)
- Names of hazardous components contributing to the classification
- UFI code (if applicable—see below)
These elements must be clearly visible on the product label. For scented products such as candles, wax melts, diffusers, and sprays, correct labelling is mandatory when the final product is classified as hazardous.
UFI Code – What You Can and Cannot Do
The UFI (Unique Formula Identifier) is a 16-digit alphanumeric code that links a hazardous mixture to a registered Poison Centre Notification (PCN). From January 1, 2025, the UFI becomes mandatory for all hazardous consumer and professional-use mixtures sold in the EU.
If you are using one of our fragrance oils without modifying it, and the final product you create is classified as hazardous under CLP, then:
✅ You must generate your own UFI and submit your own PCN for that specific mixture. Our UFI cannot be reused in this case, because the newly created product is considered a new mixture.
This also applies if you:
- Combine our fragrance oil with other hazardous substances (e.g., solvents, thinners)
- Resell or repackage or rebottle our fragrance oil
- Mix more than one fragrance oil,
- Or otherwise change the hazard profile of the final product,
If you are using one of our fragrance oils and the final product you create is does not trigger any CLP classification (meaning it is non-hazardous), then:
❌ No UFI declaration is required on the label, nor any PCN submission is required.
SDS, IFRA & Allergen Declarations
All regulatory documents (Safety Data Sheets (SDS), IFRA Certificates, and Allergen Declarations) are available for download on each product page under the “Related Documents” tab.
- Our SDS documents describe the fragrance oil as a raw material at 100% concentration.
- IFRA Certificates specify the maximum safe usage level per application type (e.g., candles, soaps, perfumes).
- Allergen declarations follow the latest EU regulation and reflect substances present above the required thresholds.
📩 Contact us if you require Certificate of Analysis documents for batch tracking.
Disclaimer
The information provided here is accurate to the best of our current knowledge and is offered in good faith. However, it only reflects the classification and regulatory contribution of the fragrance oil itself.
The ultimate legal responsibility for classifying, labelling, and notifying the final product lies with the person or business placing that product on the market. You must assess your full formulation using all available data to determine your obligations under the CLP Regulation.
Use of the CLP Generation Tool
Before using our CLP Generation Tool, you can quickly determine if your end product requires a CLP label by checking the fragrance oil’s product description. Look for the line ‘CLP declaration: >X%’. This percentage tells you the minimum concentration of fragrance needed to trigger the requirement for CLP labeling. If your usage level is below this amount, a CLP label may not be necessary for your specific product.
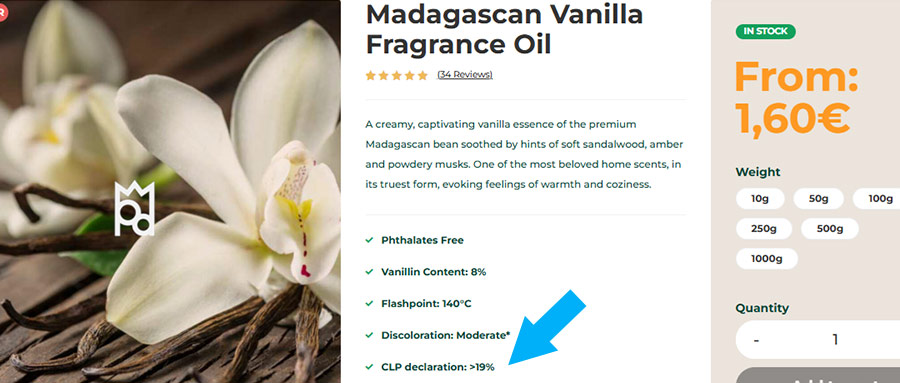
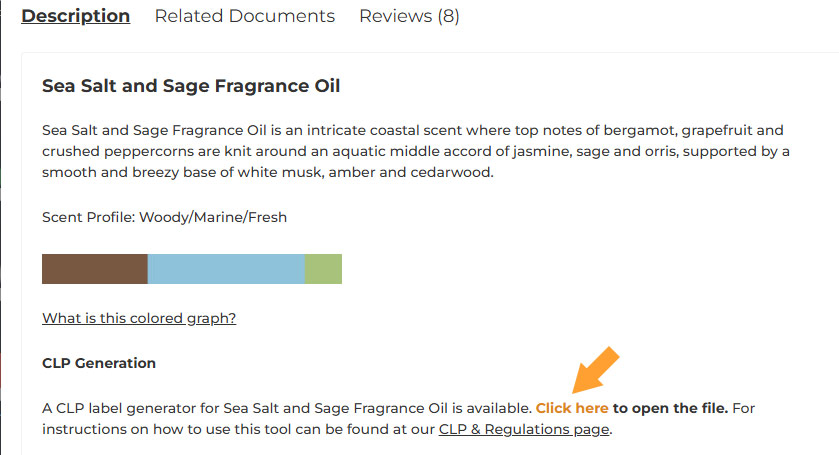
If your end product is going to have a percentage of oil higher that the declarable threshold you can find a CLP Generation Tool for each fragrance under the product’s main description. By clicking on the link you will be directed to a Google Sheets file:
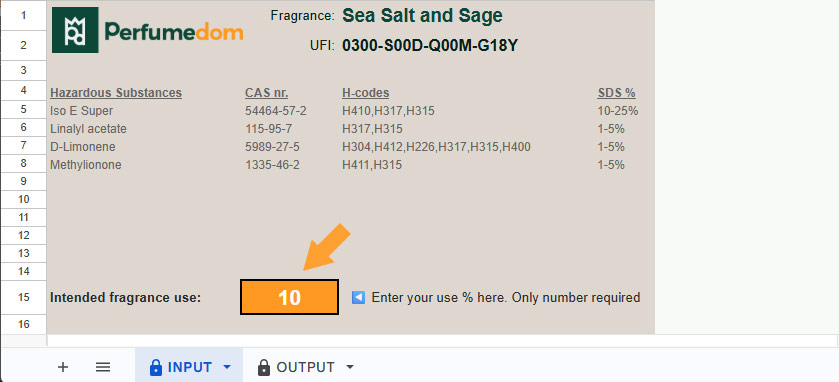
The first page (sheet) of the file is called INPUT. This is where you type the percentage of fragrance you are going to use in your final product. Note: Only a number required. Do not use the % symbol.
After typing your percentage number click on the OUTPUT page located at the bottom of the sheet.
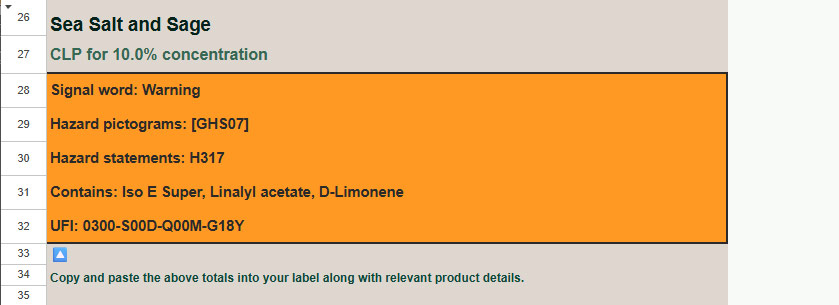
Your CLP elements have been generated! Copy all text highlighted in the orange box to your label. An appendix for all corresponding Hazard Statements and Pictograms can be found at the bottom of the page, as well as a graphic example for correct label structure.
Don’t forget to add your own UFI, your product identifier, company details, and product nominal quantity (net g or mL) for a complete and compliant label.
